In pharmaceutical chemistry, synthetic chemistry, and materials science, enantiomerically enriched alcohols serve as crucial synthetic building blocks. However, the synthesis of adjacent chiral diols remains a significant challenge. Recently, Professor Shi Lei's research group from the College of Chemistry published an important study in Angewandte Chemie International Edition, titled "Chemoselective Functionalization of Tertiary C-H Bonds of Allylic Ethers: Enantioconvergent Access to sec,tert-Vicinal Diols." The work reports a synergistic catalytic strategy combining photoredox and nickel catalysis to achieve highly chemoselective functionalization of tertiary allylic C-H bonds in allylic ethers, bypassing traditional C-O bond cleavage (Figure 1).
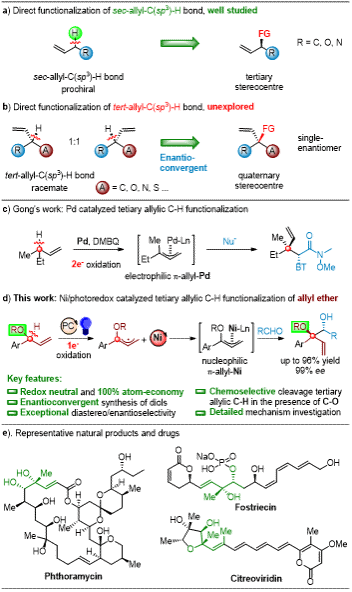
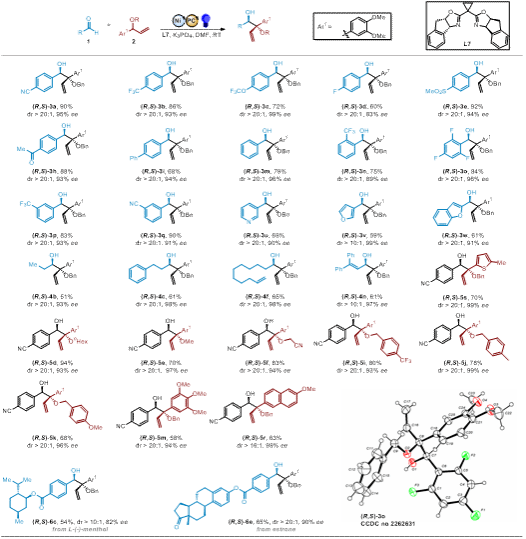
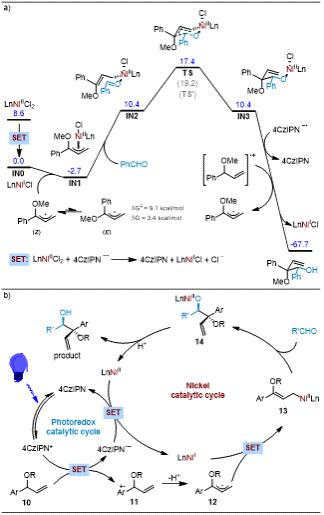
Professor Shi Lei's team developed a dual photoredox/nickel catalytic system that successfully achieved selective functionalization of tertiary C-H bonds in allylic ethers. The highlights of this work include: 1) The method exhibits excellent tolerance towards various functional groups with broad substrate scope, demonstrating outstanding enantioselectivity (up to 99% ee) and diastereoselectivity (up to 20:1 dr). 2) Mechanistic studies and density functional theory (DFT) calculations revealed that the allyl-Ni(II) species acts as a nucleophile in the carbonyl coupling reaction with aldehydes. 3) During the reaction, LnNiII undergoes single-electron transfer (SET) to form LnNiI in situ, which rapidly captures allylic radicals to generate LnNiII-allyl complexes that coordinate with benzaldehyde. The rate-determining step involves nucleophilic addition following the Zimmerman-Traxler model. The resulting nickel alkoxide then undergoes protonolysis to cleave the NiII-O bond, yielding diverse vicinal diol scaffolds. 4) Despite the low energy barrier for E/Z isomerization (9.1 kcal/mol), the Z-isomer predominates in the reaction due to its 3.4 kcal/mol thermodynamic stability advantage over the E-isomer.
The research team revealed the possible reaction mechanism through detailed mechanistic studies and density functional theory (DFT) calculations (Figure 3). First, the photoredox catalyst is excited under light irradiation to generate a strongly reducing excited state, which then produces allylic radicals via a single-electron transfer (SET) process. These radicals combine with low-valent nickel catalysts to form π-allyl-Ni intermediates. Subsequently, these intermediates undergo nucleophilic addition with aldehydes to ultimately yield the target products.
This study not only provides a novel strategy for allylic C-H functionalization, but also offers a powerful tool for synthesizing natural products and pharmaceutical molecules with complex structures. The research team successfully applied this methodology to late-stage modification of various complex molecules, demonstrating its broad application potential in medicinal chemistry and natural product synthesis. The work establishes a new pathway for selective functionalization of tertiary C-H bonds in allylic ethers, showcasing the remarkable potential of dual photoredox/nickel catalytic systems in organic synthesis. These findings are expected to advance the development of transition-metal-catalyzed methods for precise manipulation of racemic tertiary C-H bonds. This research was supported by the National Natural Science Foundation of China and other funding programs.